Adsorption glow up!
‘Pumping between phases with a pulsed-fuel molecular ratchet’ Dean Thomas, Daniel J. Tetlow, Yansong Ren, Salma Kassem, Ulvi Karaca & David A. Leigh, Nat. Nanotechnol., published online 4th April (2022). Full Article.
The sorption of species from solution into, and onto, solid phase materials is a ubiquitous process that underpins the sequestering of waste and pollutants, the recovery of precious metals, heterogeneous catalysis, many forms of chemical and biological analysis and separation science, and numerous other technologies.1,2 In such cases the transfer of the substrate between phases tends to proceed spontaneously, in the direction of equilibrium. Brownian ratchet mechanisms3,4 make it possible to drive dynamic molecular-level systems away from equilibrium.5 Now, the Leigh group report on an artificial molecular pump, immobilised on polymer beads, that uses a ratchet mechanism to actively transport, away from equilibrium, substrates from solution onto the beads.6 The molecular pump uses a pulsed chemical fuel7 that synchronizes the ratchet dynamics so that the immobilised molecular machines all act in unison to pump substrates.
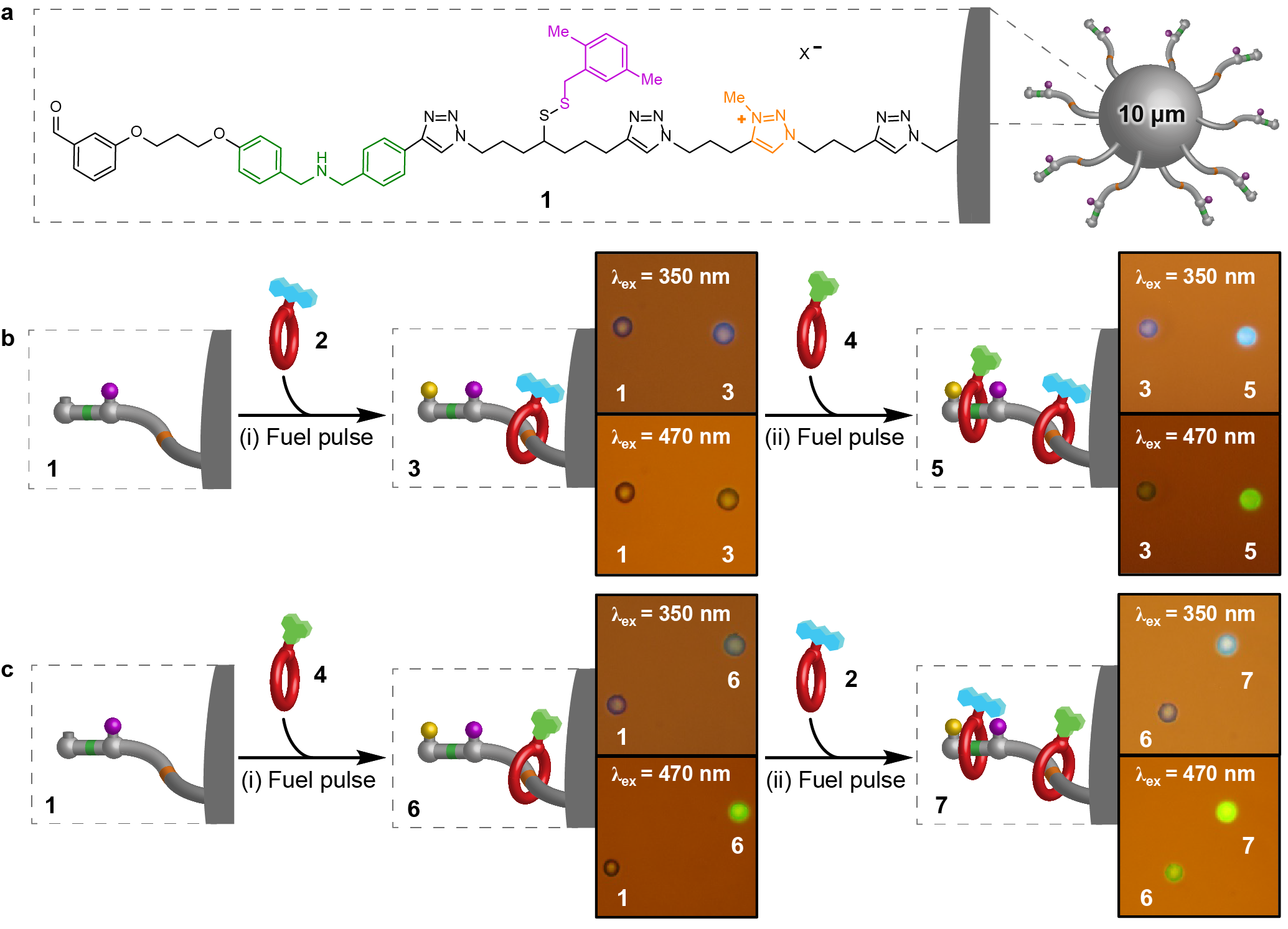
Figure 1. Out-of-equilibrium pumping from solution onto polymer beads with a pulsed-fuel molecular ratchet. a, Structure of 1. b, (i) Beads 1 with anthracene macrocycle 2 then (ii) Beads 3 with naphthalimide macrocycle 4. c, (i) Beads 1 with naphthalimide macrocycle 4 then (ii) Beads 6 with anthracene macrocycle 2. Fluorescence microscopy images comparing reacted polymer beads, excited with either 350 or 470 nm light, alongside a similar bead prior to reaction (1, 3 or 6).
Upon the addition of a pulse of CCl3CO2H fuel, micrometre-diameter polystyrene beads functionalised with an average of ~8×1010 molecular pumps per bead, sequester crown ethers appended with a fluorescent tag.6 Following consumption of the fuel, the rings are mechanically trapped in a higher energy, out-of-equilibrium, state on the beads and cannot be removed by dilution nor by switching the binding interactions off. This differs from conventional dissipative assembled materials that require a continuous supply of energy to persist.8 Addition of a second pulse of fuel causes the uptake of more macrocycles, which can be appended with a different fluorescent tag. This drives the system progressively further away from equilibrium and also confers sequence information on the deposited structure. The polymer-bound substrates (and the stored energy) can subsequently be released back to the bulk on demand, either emptying one compartment at a time or all at once (Fig. 2).
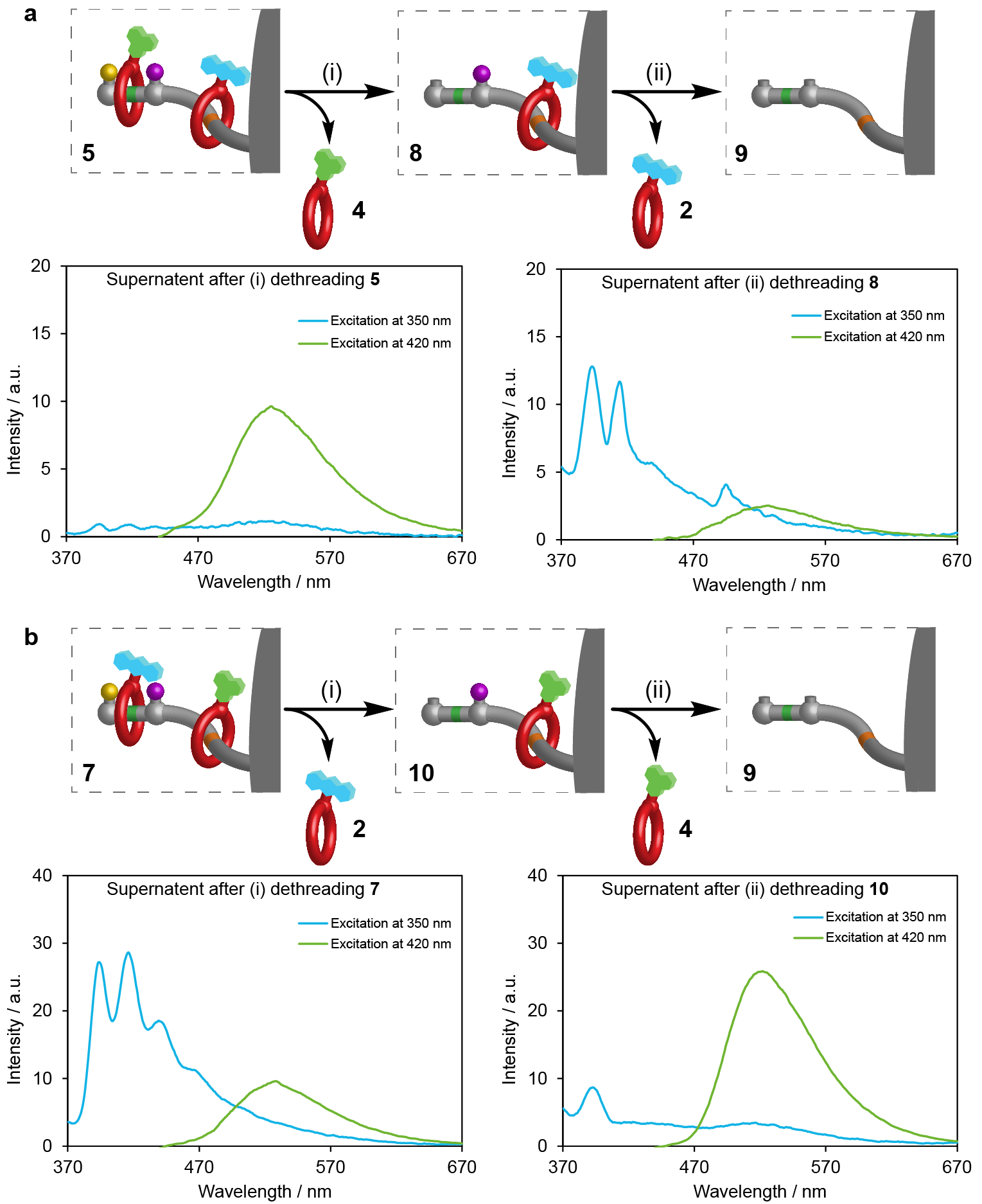
Figure 2. Reading sequence information stored in ratcheted rotaxane polymer beads. a, Operation conditions: (i) Beads 5 (0.5 mmol), methylhydrazine (200 mM), CF3CO2H (50 mM), CH3CN, r.t., 24 h. (ii) Beads 8 (0.5 mmol), DTT (50 mM), Et3N (50 mM), CH3CN, r.t., 24 h. b, Operation conditions: (i) Beads 7 (0.5 mmol), methylhydrazine (200 mM), CF3CO2H (50 mM), CH3CN, r.t., 24 h. (ii) Beads 10 (0.5 mmol), DTT (50 mM), Et3N (50 mM), CH3CN, r.t., 24 h. Fluorescence emission spectra of the corresponding supernatant acetonitrile solutions after dethreading were measured by excitation at 350 nm (blue) to detect the presence of anthracene tag 2 and 420 nm (green) to detect the presence of naphthalimide tag 4. Spectrum of acetonitrile subtracted to remove artefacts from Raman scattering.
A cartoon animation of the pumping process, showing the crown ethers with different fluorophores being pumped from solution onto polymer beads, and the sequence information being subsequently read through the release of the rings, is shown in Video 1.
The energy input for life largely originates from light (via photosynthesis). However, biology uses chemical fuels as the primary mediators of energy to power the complex nonequilibrium nanotechnology of the cell. This work demonstrates that immobilised molecular ratchets can transduce energy from a chemical fuel for the transport of substrates away from equilibrium from one phase of matter to another. It is illustrated using micron-sized polymer beads but the approach should prove applicable to other solids, surfaces, gels and nanoparticles. Indeed, a related paper from the Stoddart, Astumian and Farha groups contemporaneously reported the out-of-equilibrium adsorption of rings into MOFs by redox-driven molecular pumps, a process that the authors term ‘mechanisorption’.9
Non-equilibrium sorption through the use of immobilised artificial molecular machines that pump substrates from solution onto and into materials, has potential for the transduction of the energy from chemical fuels for the storage and release of energy and information.
References
1. Yang, Y.-W., Sun, Y.-L. & Song, N. Switchable host–guest systems on surfaces. Acc. Chem. Res. 47, 1950–1960 (2014).
2. Kolesnichenko, I. V. & Anslyn, E. V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 46, 2385–2390 (2017).
3. Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007).
4. Astumian, R. D., Mukherjee, S. & Warshel, A. The physics and physical chemistry of molecular machines. ChemPhysChem 17, 1719–1741 (2016).
5. Serreli, V., Lee, C.-F., Kay, E. R. & Leigh, D. A. A molecular information ratchet. Nature 445, 523–527 (2007).
6. Thomas, D., Tetlow, D. J., Ren, Y., Kassem, S., Karaca, U. & Leigh, D. A. Pumping between phases with a pulsed-fuel molecular ratchet. Nat. Nanotechnol. published online 4th April (2022).
7. Erbas-Cakmak, S. et al. Rotary and linear molecular motors driven by pulses of a chemical fuel. Science 358, 340–343. (2017).
8. (a) Boekhoven, J., Hendriksen, W. E., Koper, G. J. M., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015). (b) De, S. & Klajn, R. Dissipative self-assembly driven by the consumption of chemical fuels. Adv. Mater. 30, 1706750 (2018). (c) van Rossum, S. A. P., Tena-Solsona, M., van Esch, J. H., Eelkema, R. & Boekhoven, J. Dissipative out-of-equilibrium assembly of man-made supramolecular materials. Chem. Soc. Rev. 46, 5519−5535 (2017). (d) Ragazzon, G. & Prins, L. J. Energy consumption in chemical fuel-driven self-assembly. Nat. Nanotechnol. 13, 882−889 (2018). (e) Amano, S., Fielden, S. D. P. & Leigh, D. A. A catalysis-driven artificial molecular pump. Nature 594, 529–534 (2021).
9. Feng, L. et al. Active mechanisorption driven by pumping cassettes. Science 374, 1215–1221 (2021).
